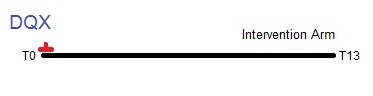Main Questionnaires
|
Participants |
Years |
|
enrollment |
close-out |
today |
| Baseline Questionnaire (BQ) |
~154,000 |
1993-2001 |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| | | | | |
| Dietary Questionnaire I-only (DQX) |
~63,000 |
1993-2001 |
|
|
|
|
|
|
|
| | | | | |
| Diet History Questionnaire (DHQ) |
~119,000 |
1998-2004 |
|
|
|
|
|
|
|
|
|
|
| | | | | |
| Supplemental Questionnaire (SQX) |
~104,000 |
2006-2007 |
|
|
|
|
|
|
|
|
|
| | | | | |
| Medical Use Questionnaire (MUQ) |
~60,000 |
2013 |
|
|
|
|
|
|
|
|
|
| | | | | |
| Brief Survey (BCQ) |
~47,000 |
2017-2018 |
|
|
|
|
|
|
|
|
|
| | | | | |
|
Cancers |
|
Active Tracking for Cancer |
State Cancer Registry Linkage* |
|
|
|
Deaths |
|
Complete Mortality Data |
|
|
* Cancer registry data is restricted, limited access only. |
Baseline (BQ)
The Baseline Questionnaire (BQ) is the baseline risk factor questionnaire and contains participant-reported information such as demographics and medical history. The form was offered to all participants, and 96.8% were completed and collected. Compliance was slightly higher in the intervention arm (97.6%) than in the control arm (96.0%). The form may have been completed before or after the participant was randomized, but most (81%) were collected within one month of randomization and nearly all (96%) were collected within three months.
Baseline Questionnaire data and documentation are incorporated into each of the
cancer datasets.
Summary
- Participant-reported data
- Covers ~150,000 participants
- Of collected forms, 98% completed between 2.3 months prior and 5.3 months after randomization
- Median time into study collected: 0 years
- Median time remaining after collection: 13 years
- Forms used:
Available Data
- Form completion details
- Demographics
- Smoking history
- Family history of cancer
- Height, weight and BMI
- NSAIDS
- Medical conditions & history of disease
- Personal history of cancer
- Female-specific information
- Male-specific information
- Screening prior to baseline
Dietary (DQX)
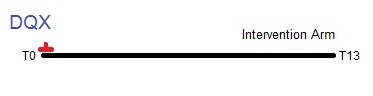
Timeline from T0 to T13 showing the collection of dietary questionnaires (DQX) in the trial. Only the intervention arm completed the DQX at T0. The control arm was not given the DQX.
The Dietary Questionnaire (DQX) was offered to intervention arm participants around the time they were randomized. 82% of the intervention arm completed a DQX. Control arm participants were not offered the DQX. The form may have been completed before (<1%) or after the participant was randomized, but about half (47%) were collected within one month of randomization and most (90%) were collected within three months.
Raw questionnaire responses were processed into analysis-ready variables in terms of gram intake, pyramid servings, food frequencies per day, etc.
DQX data can be obtained in the DQX dataset.
Summary
- Participant-reported data
- Covers ~61,000 participants
- Intervention arm only
- Of collected forms, 98% completed between day of randomization and 8.1 months after randomization
- Median time into study collected: 0 years
- Median time post-collection: 13 years
- Forms used:
Available Data
- Form completion details
- Lifestyle (alcohol use and physical activity)
- Nutrient intake
- Supplement intake
- Glycemic index/load
- Meat-specific variables
- Pyramid servings
- Daily grams of various foods and beverages
- Daily frequencies of various foods and beverages
- Raw questionnaire responses
Diet History (DHQ)
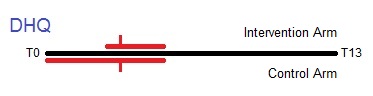
Timeline from T0 to T13 showing the collection of diet history questionnaires (DHQ) in the trial. Median completion of the DHQ was T3 for both arms of the trial, but ranges were different. Collection of DHQ from the intervention arm occurred mostly between T3 and T5, while the control arm spanned from T0 to T5.
The Diet History Questionnaire (DHQ) contains dietary information similar to that found in the DQX. 77% of all participants in both arms of the trial completed the DHQ. The form was introduced 5 years into the trial (December 1998). Raw questionnaire responses were processed into analysis-ready variables in terms of gram intake, pyramid servings, food frequencies per day, etc.
Participants in the control arm randomized prior to December 1998 were offered the DHQ in 1999 or 2000, generally around the anniversary of randomization in either year. Control arm participants randomized in/after December 1998 were offered the DHQ at baseline.
Participants in the intervention arm who were randomized prior to December 1995 were offered the DHQ in 1999, generally around the anniversary of randomization. Intervention arm participants randomized in/after December 1995 (third anniversary in/after December 1998) were offered the DHQ generally around their third anniversary of randomization (T3).
DHQ data can be obtained in the DHQ dataset.
Summary
- Participant-reported data
- Covers 113,000 participants
- Introduced in 1998
- Median time into study collected: 3 years
- Median time post-collection: 9 years
- Forms used:
Available Data
- Form completion details
- Alcohol use
- Nutrient intake
- Supplement intake
- Glycemic index/load
- Meat-specific variables
- Pyramid servings
- Daily grams of various foods and beverages
- Daily frequencies of various foods and beverages
- Raw questionnaire responses
Time of Data Acquisition

Three timelines from T0 to T13 showing the collection of diet history questionnaires (DHQ) from both arms in the trial. The first timeline is for 27,000 participants randomized before December 1995. The DHQ was completed between T4 and T6. The second timeline is for 56,000 participants randomized between December 1995 and December 1998. The DHQ was completed around T3 for both arms, but the collection from the control arm is over a slightly wider range than the intervention arm. The third timeline is for 31,000 participants randomized after December 1998. Again, most of the intervention arm completed the DHQ around T3, but the DHQ was completed by the control arm much earlier, around T0.
Supplemental (SQX)
A Supplemental Questionnaire (SQX) was introduced in 2006, 13 years into the trial. The contents of this questionnaire largely overlap with information collected on the baseline questionnaire (BQ). Some of the overlapping information is data that would not be expected to change between the BQ and the SQX (e.g. race-ethnicity). Other variables may reasonably change between the two questionnaires (e.g. marital status, smoking status, diseases). The SQX additionally contains some data not collected on the BQ (e.g., physical activity).
Ultimately, about 104,000 questionnaires were collected. Given the late administration of the SQX, the median time in the trial following a completion of this form is 3 years.
SQX data can be obtained in the SQX dataset
Summary
- Participant-reported data
- Covers ~104,000 participants
- Introduced in 2006
- Median time into study collected: 9 years
- Median time remaining after collection: 3 years
- Forms used:
Available Data
- Form completion details
- Demographics
- Family history
- BMI and physical activity
- Health history and Medication
- Smoking history
- Female-specific information
- Male-specific information
Medication Use (MUQ)
The Medication Use Questionnaire (MUQ) was administered in 2013. The questionnaire primarily asked participants about medication use. Participants were asked to provide information on aspirin, acetaminophen, and nonsteroidal anti-inflammatory drug (NSAID) use in the 12 months prior to form completion, and to list all prescription medications taken within 30 days of form completion. The MUQ also asked participants to provide their current weight and smoking status, and asked for consent to collect participants' Medicare data.
Approximately 59,000 MUQ questionnaires were collected. The median time for MUQ completion was 16 years into the trial.
MUQ data can be obtained in the Medication Use Questionnaire datasets.
Summary
- Participant-reported data
- Covers 58,895 participants
- Administered in 2013
- Median time into study collected: 16 years
- Forms used:
Available Data
- Form completion details
- Family history of cancer
- Aspirin, Acetaminophen, and NSAID usage
- Prescription drug usage
- Weight and BMI
- Smoking status
Brief Survey (BCQ)
The Brief Survey Questionnaire (BCQ) was administered between 2017 and 2018 as part of the
Buccal Cell Collection effort. The questionnaire asked participants about smoking history, height, weight,
weight loss, cancer history, physical activity, health history, cancer screening history, and gender-specific
information.
Approximately 46,600 BCQ questionnaires were collected.
The median time for BCQ completion was 20 years into the trial.
BCQ data can be obtained in the BCQ datasets.
Summary
- Participant-reported data
- Covers ~46,600 participants
- Administered in 2017 and 2018
- All questionnaires were administered after trial closeout
- Median age of participants: 80 (IQR=77-84)
- Forms used:
Available Data
- Form completion details
- Smoking status
- Height and Weight
- Weight loss
- Physical activity
- Health history
- Cancer screening history
- Male-specific information
- Female-specific information