SCU
Purpose
The Study of Colonoscopy Utilization (SCU) was an ancillary project whose primary aim was to retrospectively survey the frequency of surveillance colonoscopy –and the yield of adenoma during surveillance– in the community setting. A secondary aim was to measure the underlying prevalence of colonoscopy utilization in a smaller population. Both aims focused on the intervention arm of the PLCO screening trial. Colonoscopies recorded by SCU supplement those captured by PLCO, which were limited to procedures immediately following positive screens or immediately preceding colorectal cancer diagnoses.
Population
Approximately 5400 intervention arm participants were selected for interview. Participants were required to be living and in contact with the PLCO trial (as of 2004) and have at least five years of follow-up (i.e., entered the trial prior to September 1999). As the University of Alabama began enrollment in 1998, participants from this center were not included.
Selected participants were contacted via telephone in 2005 and 2006. Of the 5400 queried, about 5000 consented to the interview process. Of these, 4100 had colonoscopy at baseline and are therefore the focus of surveillance colonoscopy analyses. Participants with baseline adenoma or without repeat (T3/T5) screen were intentionally oversampled. Sampling weights are provided in the data.
Data Collected
The interview process attempted to ascertain whether the participant received colonoscopies not already recorded by PLCO. Participants were asked if and when colonoscopies were received, and whether any polyps or adenomas were revealed. Interview responses were captured on a Colonoscopy Utilization Questionnaire (CQX). The CQX template is available on this site.
For participants who reported an additional colonoscopy, medical records were reviewed. All colonoscopies found during review were captured by SCU regardless of the accuracy of self-report, excluding those previously known by PLCO. These new colonoscopies were abstracted on the third version of the Diagnostic Evaluation – Colorectum form (DEC3).
Data collected in SCU has limited scope if not combined with information from the PLCO database. Therefore, the data available for download combines both databases to provide a complete history of colonoscopy utilization for each selected participant. Censoring dates have been built and included to identify time beyond which complete colonoscopy utilization is unknown.
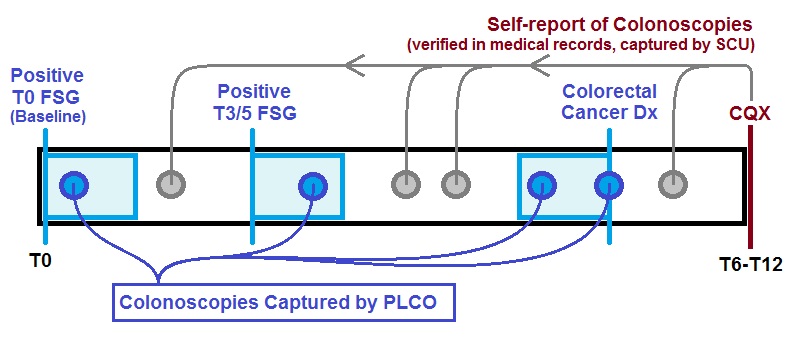
This timeline shows how colonoscopies were identified for a generic participant in the SCU population. Colonoscopies immediately following positive screens or immediately preceding colorectal cancer diagnoses were captured by PLCO. SCU captured all other colonoscopies if the participant reported any additional colonoscopies at interview, which took place anywhere from 6 to 12 years into the trial.
SCU data for the primary aim of the SCU study (surveillance colonoscopy utilization and yield) can be obtained in the SCU dataset .
Summary
- Retrospective survey of colonoscopies outside of the standard PLCO data collection
- Data collected from medical records and participant reports
- Covers 4100 intervention arm participants
- Most interviews completed in 2005
- Median time into study collected: 9.5 years (IQR: 8.8-10.1 years)
- Forms used:
- CQX (PDF - 299 KB)
Available Data
- PLCO and SCU-captured colonoscopies
- Baseline versus surveillance colonoscopy
- Findings on colonoscopy (e.g., adenoma)
- Self-reports of colonoscopy and findings
- Days from randomization to CQX interview
- Days from randomization to colonoscopies and colonoscopy censoring date
- Sampling weights
Main Findings
-
Utilization of surveillance colonoscopy in community practice.
Schoen RE, Pinsky PF, Weissfeld JL, Yokochi LA, Reding DJ, Hayes RB, Church T, Yurgalevich S, Doria-Rose VP, Hickey T, Riley T, Berg CD
Gastroenterology. 2010 Jan; Volume 138 (Issue 1): Pages 73-81 PUBMED -
The yield of surveillance colonoscopy by adenoma history and time to examination.
Pinsky PF, Schoen RE, Weissfeld JL, Church T, Yokochi LA, Doria-Rose VP, Prorok P
Clin. Gastroenterol. Hepatol. 2009 Jan; Volume 7 (Issue 1): Pages 86-92 PUBMED
